Lista de elementos
»
actínio
»
alumínio
»
amerício
»
argão
»
arsênico
»
astatine
»
azoto
»
bário
»
Berílio
»
bismuto
»
bohrium
»
Boro
»
bromo
»
cádmio
»
cálcio
»
carbono
»
cério
»
césio
»
cloro
»
cobalto
»
cobre
»
crômio
»
dubnium
»
enxofre
»
erbium
»
escândio
»
estanho
»
európio
»
fermium
»
ferro
»
fleróvio
»
flúor
»
fósforo
»
francium
»
gálio
»
germânio
»
háfnio
»
hassium
»
hélio
»
holmium
»
índio
»
iodo
»
irídio
»
itérbio
»
ítrio
»
lantânio
»
lead
»
lítio
»
lutécio
»
magnésio
»
manganês
»
mercúrio
»
neodímio
»
néon
»
neptúnio
»
Nihonium
»
nióbio
»
níquel
»
nobelium
»
ósmio
»
ouro
»
oxigênio
»
paládio
»
platina
»
plutônio
»
polônio
»
potássio
»
Prata
»
promécio
»
radão
»
rádio
»
rênio
»
ródio
»
rubídio
»
rutênio
»
samário
»
selênio
»
silício
»
sódio
»
tálio
»
tântalo
»
tecnécio
»
telúrio
»
Tennesse
»
térbio
»
titânio
»
tório
»
túlio
»
urânio
»
vanádio
»
xênon
»
zinco
»
zircônio
mineralogia
elementos
Sb antimônio
Sb - antimônio - METALÓIDES
O antimônio é um elemento químico metálico, denotado Sb e com número atômico 51, que faz parte do grupo 15 da tabela periódica dos elementos. É um metal branco brilhante, que se transforma em um pó incolor quando exposto ao ar.
As propriedades físicas do antimônio são: Densidade: 6,69 g/cm3, ponto de fusão: 630°C, ponto de ebulição: 1587°C e ponto de fulgor: 630°C.
O antimônio possui propriedades químicas interessantes como: é muito reativo com ácidos, dissolvendo-se facilmente em soluções ácidas; pode reagir vigorosamente com halogênios; e reage com oxigênio, halogênios e sais para formar compostos oxoácidos.
O antimônio é usado principalmente para fazer ligas metálicas, baterias, semicondutores e catalisadores. Também é freqüentemente usado na indústria têxtil, indústria química e indústria de corantes. Compostos de antimônio também são usados como inseticidas e fungicidas.
As propriedades físicas do antimônio são: Densidade: 6,69 g/cm3, ponto de fusão: 630°C, ponto de ebulição: 1587°C e ponto de fulgor: 630°C.
O antimônio possui propriedades químicas interessantes como: é muito reativo com ácidos, dissolvendo-se facilmente em soluções ácidas; pode reagir vigorosamente com halogênios; e reage com oxigênio, halogênios e sais para formar compostos oxoácidos.
O antimônio é usado principalmente para fazer ligas metálicas, baterias, semicondutores e catalisadores. Também é freqüentemente usado na indústria têxtil, indústria química e indústria de corantes. Compostos de antimônio também são usados como inseticidas e fungicidas.
Sintético
Radioativo
Líquido
Gasoso
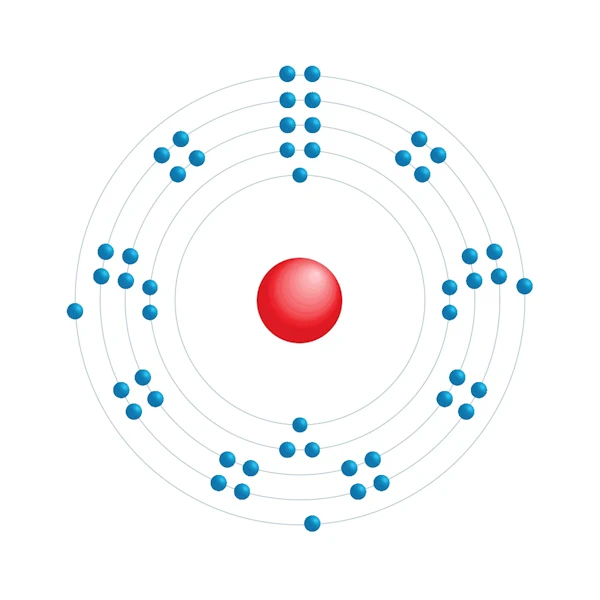
Diagrama de configuração eletrônica
| Nome | antimônio |
| Número | 51 |
| Atômico | 121.76 |
| Símbolo | Sb |
| Fusão | 630.7 |
| Ebulição | 1750 |
| Densidade | 6.685 |
| Período | 5 |
| Grupo | 15 |
| Descoberta | 0 Early historic times |
| Abundância | 0.2 |
| Raio | 1.5 |
| Eletronegatividade | 2.05 |
| Ionização | 8.6084 |
| Número de isótopos | 29 |
| Configuração eletrônica | [Kr] 4d10 5s2 5p3 |
| Estados de oxidação | -3,3,5 |
| Elétron por nível de energia | 2,8,18,18,5 |
| Mineral | Dureza | Densidade |
| Allargentum | 4.00 / 4.00 | 10.00 |
| Ambrinoite | 3.31 | |
| Andorite IV | 3.50 / 3.50 | 5.00 |
| Andorite VI | 3.50 / 3.50 | 5.00 |
| Andreadiniite | ||
| Antimonselite | 3.50 / 3.50 | 5.88 |
| Anyuiite | 3.50 / 3.50 | 13.00 |
| Apuanite | 4.00 / 5.00 | 5.33 |
| Aramayoite | 2.50 / 2.50 | 5.60 |
| Ardaite | 2.50 / 3.00 | 6.00 |
| Argentotennantite | 3.50 / 3.50 | 5.00 |
| Argentotetrahedrite | ||
| Arsenopalladinite | 4.00 / 4.00 | 10.40 |
| Arsenpolybasite | 3.00 / 3.00 | 6.18 |
| Arsenquatrandorite | ||
| Auroantimonate | 4.00 / 4.00 | |
| Aurostibite | 3.00 / 3.00 | 9.98 |
| Bahianite | 9.00 / 9.00 | 4.89 |
| Barikaite | 5.34 | |
| Baumstarkite | 2.50 / 2.50 | 5.33 |
| Benavidesite | 2.50 / 2.50 | 5.60 |
| Benleonardite | 3.50 / 3.50 | 7.00 |
| Bernardite | 2.00 / 2.00 | 4.50 |
| Bernarlottiite | ||
| Berthierite | 2.00 / 2.50 | 4.00 |
| Biehlite | 1.00 / 1.50 | |
| Billwiseite | 5.00 / 5.00 | 6.33 |
| Bindheimite | 4.00 / 5.00 | 4.60 |
| Bismutostibiconite | 4.00 / 5.00 | 7.38 |
| Bitikleite | ||
| Blatterite | 6.00 / 6.00 | 4.70 |
| Borodaevite | 3.50 / 3.50 | 7.90 |
| Borovskite | 3.00 / 3.00 | 8.12 |
| Boscardinite | 3.00 | |
| Bottinoite | 3.50 / 3.50 | 2.83 |
| Boulangerite | 2.50 / 2.50 | 5.70 |
| Bournonite | 3.00 / 3.00 | 5.70 |
| Braithwaiteite | 2.00 / 2.00 | 3.75 |
| Brandholzite | 2.00 / 3.00 | |
| Breithauptite | 3.50 / 4.00 | 8.23 |
| Brizziite | 2.50 / 2.50 | 4.00 |
| Byströmite | 7.00 / 7.00 | 5.70 |
| Camérolaite | 3.10 | |
| Carducciite | 2.50 / 3.00 | 5.56 |
| Cervantite | 4.00 / 5.00 | 6.40 |
| Cesplumtantite | 7.00 / 7.00 | 6.00 |
| Cetineite | 3.50 / 3.50 | 4.00 |
| Chabournéite | 3.00 / 3.50 | 5.10 |
| Chalcostibite | 3.50 / 3.50 | 4.75 |
| Chalcothallite | 2.00 / 2.50 | 6.60 |
| Chapmanite | 2.50 / 2.50 | 3.58 |
| Chestermanite | 6.00 / 6.00 | 3.72 |
| Choloalite | 3.00 / 3.00 | 6.40 |
| Chovanite | 3.00 / 3.00 | 6.03 |
| Ciriottiite | ||
| Clerite | 3.50 / 4.00 | 4.93 |
| Clino-oscarkempffite | ||
| Clinocervantite | ||
| Colusite | 3.00 / 4.00 | 4.20 |
| Coquandite | 3.00 / 4.00 | 5.00 |
| Costibite | 6.00 / 6.00 | 6.89 |
| Criddleite | 3.00 / 3.50 | 6.00 |
| Cualstibite | 2.00 / 2.00 | 3.18 |
| Cuboargyrite | 3.00 / 3.00 | 5.33 |
| Cupropolybasite | 3.00 / 3.50 | 6.31 |
| Cuprostibite | 4.00 / 4.00 | 8.42 |
| Cyanophyllite | 2.00 / 2.00 | 3.10 |
| Cylindrite | 2.50 / 2.50 | 5.40 |
| Dadsonite | 2.50 / 2.50 | 5.68 |
| Dalnegroite | 3.00 / 3.50 | |
| Derbylite | 5.00 / 5.00 | 4.50 |
| Diaphorite | 2.50 / 2.50 | 6.00 |
| Disulfodadsonite | ||
| Dyscrasite | 3.50 / 4.00 | 9.40 |
| Dzhuluite | 4.71 | |
| Ecrinsite | ||
| Falkmanite | 2.00 / 3.00 | 6.31 |
| Famatinite | 3.00 / 4.00 | 4.57 |
| Ferdowsiite | 2.50 / 3.00 | 5.30 |
| Fettelite | 3.50 / 4.00 | 6.29 |
| Filipstadite | 6.00 / 6.50 | 4.00 |
| Fizélyite | 2.00 / 2.00 | 5.56 |
| Florensovite | 5.00 / 5.00 | 4.00 |
| Fluorcalcioroméite | 5.50 / 5.50 | 5.11 |
| Folvikite | ||
| Franckeite | 2.50 / 2.50 | 5.50 |
| Freibergite | 3.50 / 4.00 | 4.85 |
| Freieslebenite | 2.50 / 2.50 | 6.20 |
| Fülöppite | 2.00 / 2.50 | 5.22 |
| Gabrielite | 1.50 / 2.00 | 5.38 |
| Galkhaite | 3.00 / 3.00 | 5.40 |
| Garavellite | 4.00 / 4.00 | 5.64 |
| Genkinite | 5.50 / 6.00 | 8.83 |
| Geocronite | 2.50 / 3.00 | 6.40 |
| Gerstleyite | 2.50 / 2.50 | 3.62 |
| Getchellite | 1.50 / 2.00 | 3.92 |
| Geversite | 4.50 / 5.00 | 10.97 |
| Giessenite | 2.50 / 2.50 | 7.45 |
| Gillulyite | 2.00 / 2.50 | 4.02 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se