Lista de elementos
»
actínio
»
alumínio
»
amerício
»
argão
»
arsênico
»
astatine
»
azoto
»
bário
»
Berílio
»
bismuto
»
bohrium
»
Boro
»
bromo
»
cádmio
»
cálcio
»
carbono
»
cério
»
césio
»
cloro
»
cobalto
»
cobre
»
crômio
»
dubnium
»
enxofre
»
erbium
»
escândio
»
estanho
»
európio
»
fermium
»
ferro
»
fleróvio
»
flúor
»
fósforo
»
francium
»
gálio
»
germânio
»
háfnio
»
hassium
»
hélio
»
holmium
»
índio
»
iodo
»
irídio
»
itérbio
»
ítrio
»
lantânio
»
lead
»
lítio
»
lutécio
»
magnésio
»
manganês
»
mercúrio
»
neodímio
»
néon
»
neptúnio
»
Nihonium
»
nióbio
»
níquel
»
nobelium
»
ósmio
»
ouro
»
oxigênio
»
paládio
»
platina
»
plutônio
»
polônio
»
potássio
»
Prata
»
promécio
»
radão
»
rádio
»
rênio
»
ródio
»
rubídio
»
rutênio
»
samário
»
selênio
»
silício
»
sódio
»
tálio
»
tântalo
»
tecnécio
»
telúrio
»
Tennesse
»
térbio
»
titânio
»
tório
»
túlio
»
urânio
»
vanádio
»
xênon
»
zinco
»
zircônio
mineralogia
elementos
Sn estanho
Sn - estanho - METAL POBRE
O estanho é um elemento químico metálico, de símbolo químico Sn e de número atómico 50. Faz parte do grupo 14 da tabela periódica dos elementos, reputado pertencer à família dos metais de transição, nomeadamente os que se encontram entre os metais e não -metais na tabela.
Pewter é encontrado em seu estado nativo, bem como em uma liga. É amplamente utilizado na indústria por suas propriedades metalúrgicas e mecânicas.
O estanho é um metal cinza prateado muito maleável e maleável. Quebra facilmente e torce facilmente sob a ação de forças mecânicas. Possui baixa densidade em relação a outros metais, além de excelente condutividade elétrica. Finalmente, tem um ponto de fusão de 232°C.
O estanho é um metal muito estável, conhecido por ser muito refratário a ácidos e corrói com dificuldade, o que o torna um metal muito procurado por sua resistência à corrosão. Além disso, possui boa condutividade térmica e é altamente resistente ao calor e à oxidação.
O estanho é amplamente utilizado na indústria, por suas propriedades metalúrgicas e suas propriedades mecânicas. É especialmente utilizado para fabricar componentes eletrônicos, peças mecânicas e produtos eletromecânicos, baterias, acessórios e materiais de isolamento. Finalmente, é usado para produzir cosméticos e produtos de limpeza doméstica, além de tintas e tintas.
Pewter é encontrado em seu estado nativo, bem como em uma liga. É amplamente utilizado na indústria por suas propriedades metalúrgicas e mecânicas.
O estanho é um metal cinza prateado muito maleável e maleável. Quebra facilmente e torce facilmente sob a ação de forças mecânicas. Possui baixa densidade em relação a outros metais, além de excelente condutividade elétrica. Finalmente, tem um ponto de fusão de 232°C.
O estanho é um metal muito estável, conhecido por ser muito refratário a ácidos e corrói com dificuldade, o que o torna um metal muito procurado por sua resistência à corrosão. Além disso, possui boa condutividade térmica e é altamente resistente ao calor e à oxidação.
O estanho é amplamente utilizado na indústria, por suas propriedades metalúrgicas e suas propriedades mecânicas. É especialmente utilizado para fabricar componentes eletrônicos, peças mecânicas e produtos eletromecânicos, baterias, acessórios e materiais de isolamento. Finalmente, é usado para produzir cosméticos e produtos de limpeza doméstica, além de tintas e tintas.
Sintético
Radioativo
Líquido
Gasoso
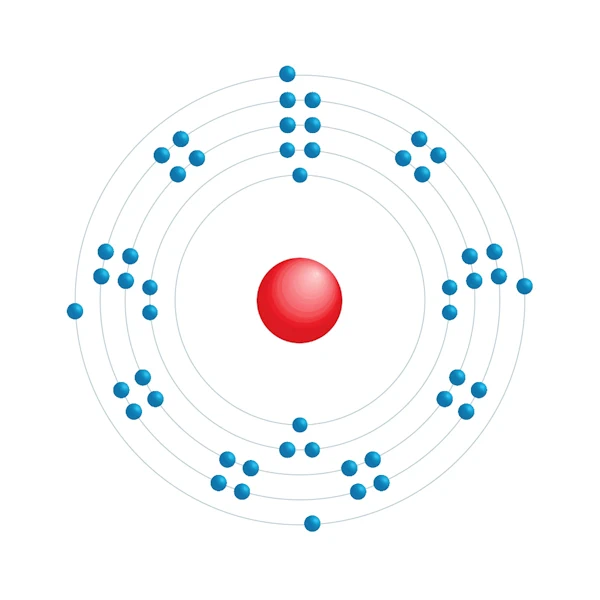
Diagrama de configuração eletrônica
| Nome | estanho |
| Número | 50 |
| Atômico | 118.71 |
| Símbolo | Sn |
| Fusão | 232 |
| Ebulição | 2270 |
| Densidade | 7.287 |
| Período | 5 |
| Grupo | 14 |
| Descoberta | 0 Prehistoric |
| Abundância | 2.3 |
| Raio | 1.7 |
| Eletronegatividade | 1.96 |
| Ionização | 7.3439 |
| Número de isótopos | 28 |
| Configuração eletrônica | [Kr] 4d10 5s2 5p2 |
| Estados de oxidação | -4,2,4 |
| Elétron por nível de energia | 2,8,18,18,4 |
| Mineral | Dureza | Densidade |
| Abhurite | 2.00 / 2.00 | 4.29 |
| Abramovite | ||
| Agmantinite | ||
| Aleksandrovite | 4.00 / 4.50 | 3.07 |
| Aluminomagnesiohulsite | 6.00 / 6.00 | 3.84 |
| Asbecasite | 6.50 / 7.00 | 3.70 |
| Atokite | 5.00 / 5.00 | 14.90 |
| Berndtite | 1.00 / 2.00 | 4.50 |
| Bitikleite | ||
| Brannockite | 5.00 / 6.00 | 2.98 |
| Burtite | 3.00 / 3.00 | 3.28 |
| Butianite | ||
| Cabriite | 4.00 / 4.50 | 10.70 |
| Canfieldite | 2.50 / 2.50 | 6.28 |
| Cassiterite | 6.00 / 7.00 | 6.80 |
| Cernýite | 4.00 / 4.00 | 4.78 |
| Cesplumtantite | 7.00 / 7.00 | 6.00 |
| Chatkalite | 4.50 / 4.50 | 5.00 |
| Coiraite | 2.00 / 2.00 | 5.92 |
| Colusite | 3.00 / 4.00 | 4.20 |
| Cylindrite | 2.50 / 2.50 | 5.40 |
| Dzhuluite | 4.71 | |
| Eakerite | 5.50 / 5.50 | 2.93 |
| Erazoite | ||
| Erniggliite | 2.00 / 3.00 | 5.00 |
| Eta - bronze | ||
| Ferrohögbomite-2N2S | 6.00 / 7.00 | |
| Ferrokësterite | 4.00 / 4.00 | 4.00 |
| Ferronigerite-2N1S | 8.50 / 8.50 | 4.51 |
| Ferronigerite-6N6S | 8.50 / 8.50 | 4.51 |
| Ferrowodginite | 5.50 / 5.50 | 7.00 |
| Foordite | 6.00 / 6.00 | 6.73 |
| Franckeite | 2.50 / 2.50 | 5.50 |
| Genplesite | ||
| Hemusite | 4.00 / 4.00 | 4.47 |
| Herzenbergite | 2.00 / 2.00 | 5.20 |
| Hocartite | 4.00 / 4.00 | 4.77 |
| Hulsite | 3.00 / 3.00 | 4.30 |
| Hydroromarchite | 5.00 | |
| Incaite | 2.00 / 2.00 | |
| Irinarassite | 4.30 | |
| Ixiolite | 6.00 / 6.50 | 6.94 |
| Jeanbandyite | 3.50 / 3.50 | 4.41 |
| Kësterite | 4.50 / 4.50 | 4.54 |
| Kiddcreekite | 4.00 / 4.00 | 4.00 |
| Kojonenite | ||
| Kristiansenite | 5.50 / 6.00 | 3.30 |
| Kuramite | 5.00 / 5.00 | 4.56 |
| Lakargiite | 8.00 / 8.50 | 4.59 |
| Lévyclaudite | 2.50 / 3.00 | 6.00 |
| Magnesiohulsite | 5.50 / 6.50 | 4.18 |
| Magnesionigerite-2N1S | 8.00 / 8.50 | 4.22 |
| Magnesionigerite-6N6S | 8.00 / 8.50 | 4.22 |
| Malayaite | 3.50 / 4.00 | 4.30 |
| Mawsonite | 3.50 / 4.00 | 4.66 |
| Megawite | 5.06 | |
| Mengxianminite | 6.00 / 6.00 | 3.85 |
| Mohite | 4.00 / 4.00 | 4.86 |
| Mushistonite | 4.00 / 4.50 | 4.04 |
| Nalivkinite | 3.29 | |
| Natanite | 5.00 / 5.00 | 4.04 |
| Nekrasovite | 4.50 / 5.00 | 4.62 |
| Niggliite | 3.00 / 3.00 | 13.44 |
| Nisnite | 9.41 | |
| Nordenskiöldine | 5.50 / 6.00 | 4.20 |
| Ottemannite | 2.00 / 2.00 | 4.84 |
| Oulankaite | 3.50 / 4.00 | 10.27 |
| Ovamboite | 3.50 / 3.50 | 4.74 |
| Oxystannomicrolite | ||
| Pabstite | 6.00 / 6.00 | 4.03 |
| Palarstanide | 5.00 / 5.00 | 10.00 |
| Panichiite | 2.43 | |
| Paolovite | 5.00 / 5.00 | 11.32 |
| Petrukite | 4.50 / 4.50 | 4.61 |
| Pirquitasite | 4.00 / 4.00 | 4.82 |
| Potosíite | 2.50 / 2.50 | 6.20 |
| Rhodostannite | 4.00 / 4.00 | 4.00 |
| Romarchite | 2.00 / 2.50 | |
| Rustenburgite | 5.00 / 5.00 | 15.00 |
| Sakuraiite | 4.00 / 4.00 | 4.00 |
| Samarskite-(Yb) | 5.00 / 6.00 | 7.03 |
| Schoenfliesite | 4.00 / 4.50 | 3.48 |
| Skaergaardite | 4.00 / 5.00 | 10.64 |
| Sorosite | 5.00 / 5.50 | 7.60 |
| Stannite | 3.50 / 4.00 | 4.30 |
| Stannoidite | 4.00 / 4.00 | 4.29 |
| Stannopalladinite | 5.00 / 5.00 | 10.20 |
| Stibiocolusite | 4.00 / 4.50 | 4.00 |
| Stistaite | 3.00 / 3.00 | 6.91 |
| Stokesite | 6.00 / 6.00 | 3.20 |
| Suredaite | 2.50 / 3.00 | 5.54 |
| Sverigeite | 6.50 / 6.50 | 3.60 |
| Sørensenite | 5.50 / 5.50 | 2.90 |
| Taimyrite II | ||
| Taimyrite-I | 5.00 / 5.00 | |
| Taseqite | 5.50 / 5.50 | 3.24 |
| Tatyanaite | 3.50 / 4.00 | |
| Teallite | 1.50 / 2.00 | 6.40 |
| Tellurocanfieldite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se